
Omega-3 and Omega-6 Fatty Acids
There has been much talk about omega-3 and omega-6 fatty acids, and their potential benefits. Many people also know that trans-fatty acids are harmful. However, the discussion of fats is a complex subject. Lipids can exist in the human body as sterols, phospholipids, triglycerides, or free fatty acids. However, we will focus on the fatty acids that make free fatty acids, triglycerides, and phospholipids.
Structure of Fatty Acids
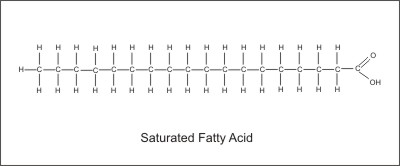
The molecular structure is the main factor that determines the nature and type of fatty acid. A fatty acid consists of a hydrocarbon chain with a carboxyl (COOH) group at one end. The carboxyl group is acidic (Hence the term "fatty acid"). The molecular structure of the fatty acid is determined by the number of carbons in the chain, the presence and number of missing hydrogen pairs, the relative positions of these missing pairs, and the orientations of the hydrogen atoms. These factors determine characteristics such as the melting point, the shape, and the level of polarization of the fat molecule. For example, a saturated fat is defined as having no missing hydrogen pairs which means that the molecule is saturated with hydrogen.
Although many saturated fats are solid at room temperature, this is not the defining characteristic. A fatty acid with one missing hydrogen pair is a mono-unsaturated fat, while a fatty acid with 2 or more missing hydrogen pairs is a poly-unsaturated fat. The convention for labeling natural fatty acids is such that the first number represents the number of carbon atoms, the second number represents the number of missing pairs, and the third number tells the position of the first missing pair. Also, the term "Cis" or "Trans" at the beginning tells whether the functional groups are on the same side (Cis) or across from each other (Trans). For example:
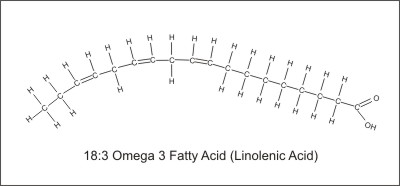
Cis-18:3 omega 3 means there are 18 carbon atoms and 3 missing hydrogen pairs starting at the 3rd carbon from the hydrocarbon end. The word "Cis" means that the functional groups are on the same side of the molecule. This is an omega-3 fatty acid called linolenic acid.
Cis-18:2 omega 6 means there are 18 carbon atoms and 2 missing hydrogen pairs starting at the 6th carbon from the hydrocarbon end. The "Cis" means that the functional groups are on the same side of the molecule. This is an omega-6 fatty acid called linoleic acid.
Trans-18:2 omega 6 means there are 18 carbon atoms and 2 missing hydrogen pairs starting at the 6th carbon from the hydrocarbon end. The word "Trans" (Latin for across) means that the functional groups are across from each other. This is a trans-fatty acid that is an isomer of linoleic acid.
Functions of Essential Fats and What Makes Them Essential
The natural unsaturated fats have the missing pairs of hydrogen atoms on the same side of the molecule (cis configuration). Because the hydrogen atoms share their electrons with the carbon atoms, they have a slight positive charge and therefore repel each other. This bends the cis-configured fatty acid into a distinct shape and polarizes the molecule, which alters its electrical properties. The body can remove hydrogen pairs from the fatty acids but cannot remove the hydrogen pairs from the 3rd or the 6th positions. So, omega 3 and omega 6 fatty acids are essential because we need fats with the hydrogen pairs already removed from these positions. This makes them nutrients, so we must eat foods that have omega-3 and omega-6 fatty acids. These fatty acids, along with the highly unsaturated fatty acids that are made from them, have many functions in the body which include but are not limited to the following:
1. Transporting oxygen from the lungs to the red blood cells
2. Regulating cell division and gene expression
3. Development of the central nervous system
4. Manufacture of prostaglandins which have many functions in the body
5. Structural components of cell membranes
Effects of distorted fats and what causes them
Unsaturated fatty acids can be distorted if they are subjected to extreme conditions. These conditions can cause the fatty acid molecules to twist around so that the hydrogen atoms surrounding the missing hydrogen pairs are across from each other. This configuration is known as the trans (Latin for across) configuration and will alter the shape, the melting point, and the polarization of the molecule. This will adversely affect its function.
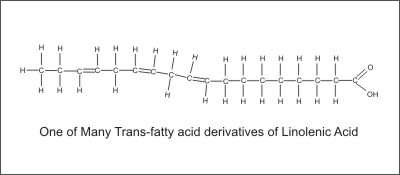
The body will often try to use trans-fatty acids for functions that are best fulfilled by the cis-fatty acids. This often leads to bad results that can cause long-term health problems. Although the production of trans-fatty acids is the most well-known result of distorting fatty acids, there are other potentially bad results such as bond shifting, abnormal conjugations, and other effects which are yet to be studied. The distortion of unsaturated fats can result from rancidity as well as from excessive heating and processing. This is especially true for partial hydrogenation where hydrogen is forced into the fat molecules under extreme heat and pressure.
Although many of the effects of essential fatty acids are yet to be understood, it is clear that they are necessary in the diet. It is equally clear that getting adequate amounts of essential fatty acids means eating the right type of foods as opposed to just eating larger quantities of greasy junk. It is equally necessary to avoid many of the distorted fats including trans-fatty acids which are present in deep-fried foods, processed cooking oils, and partially hydrogenated oils such as margarines.
Other articles